Shortest time ever measured
In 1999, Egyptian chemist Ahmed Zewail received the Nobel Prize for measuring the speed at which molecules change shape.
He founded femto-chemistry using ultrashort laser flashes: The formation and breaking of chemical bonds occurs on the femtosecond scale – a femtosecond is equal to 0.000000000000001 second, or 10 -15 seconds.
Ten years later, a German team measured time on the attosecond scale – 1 attosecond equals 10 -18 seconds.
Now, a decade more, another team of German physicists measured for the first time a process on the zeptosecond scale – 1 zeptosecond equals 10 -21 seconds -, again breaking the record for the shortest time ever measured.
Sven Grundmann and his colleagues at Goethe University measured how long it takes for a photon to cross a hydrogen molecule: About 247 zeptoseconds for the average length of the molecule’s bond.
Time at the atomic level
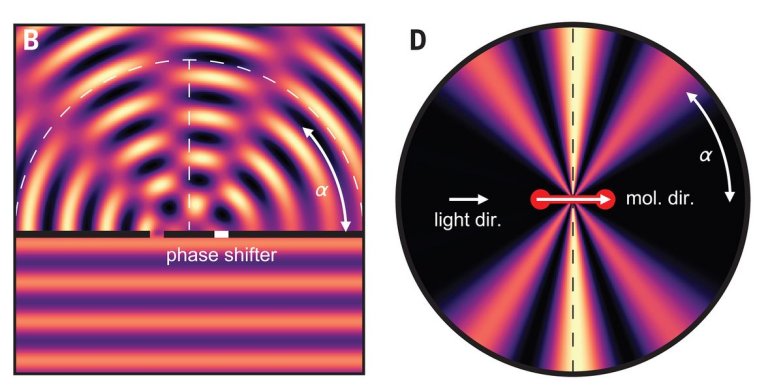
The team measured time on a hydrogen (H2) molecule that received an X-ray shot from the synchrotron light source of the DESY accelerator. They adjusted the X-ray energy so that one photon was enough to eject the two electrons in the hydrogen molecule.
Electrons behave like particles and waves simultaneously, and therefore the ejection of the first electron resulted in waves of electrons launched first in one, and then in the second atom of the hydrogen molecule, in rapid succession, with the waves merging.
The photon behaved in a very similar way to a flat stone thrown on a lake, bouncing and bouncing twice: When a wave valley encounters a crest of another wave, the waves of the first and second electrons cancel each other, resulting in what is called an interference pattern.
The team measured the interference pattern of the first electron ejected simultaneously with the interference pattern, which allowed to determine the orientation of the hydrogen molecule – they took advantage of the fact that the second electron also left the hydrogen molecule, so that the the remaining hydrogen nucleus “went flying”, which allowed for its detection.
Light interacting with molecule
“As we knew the spatial orientation of the hydrogen molecule, we used the interference of the two electron waves to calculate precisely when the photon reached the first and when it reached the second hydrogen atom,” explained Grundmann. “And it lasts up to 247 zeptoseconds, depending on how far apart in the molecule the two atoms were from the perspective of light.”
“We observed for the first time that the electron layer in a molecule does not react to light everywhere at the same time. The delay occurs because the information inside the molecule only spreads at the speed of light,” said Professor Reinhard Dörner.Bibliography:
Article: Zeptosecond Birth Time Delay in Molecular Photoionization
Authors: Sven Grundmann, Daniel Trabert, Kilian Fehre, Nico Strenger, Andreas Pier, Leon Kaiser, Max Kircher, Miriam Weller, Sebastian Eckart, Lothar Ph. H. Schmidt, Florian Trinter, Till Jahnke, Markus S. Schöffler, Reinhard Dörner
Magazine: Science
Vol .: 370, Issue 6514, pp. 339-341
DOI: 10.1126 / science.abb9318































Discussion about this post