Superconductivity
Physicists from three North American universities have accomplished a long-awaited feat: They have demonstrated superconductivity at room temperature.
Until today, lossless electricity transfer had only been achieved at cryogenic temperatures.
It is not yet the expected revolution in the way we use energy because the feat was achieved only under extremely high pressure.
However, although the phenomenon observed by the team is at an early stage, or fundamental level, the discovery has implications for how energy is stored and transmitted, reinforcing the expectation that superconductivity may one day change the way all electronic devices are how people and cargo are transported and how the whole of society can achieve a new level of energy efficiency.
Metallic hydrogen
Since their first observation, in 1911, scientists have made superconductivity work only at very low temperatures, close to absolute zero (-273 ºC), which would make its practical and widespread application unattainable.
But progress has been made, until the discovery of what are now called “high-temperature superconductors” – in fact, their temperature is very low, around -170 ºC -, which enabled the use of technology on several fronts, of magnetic resonance equipment to particle accelerator supermagnets like the LHC.
But as early as 1968, physicists predicted that metallic hydrogen – which can only be obtained at very high pressures – could be the key ingredient in discovering superconductivity at room temperature or even higher.
Therefore, Elliot Snider and his colleagues began to try to synthesize metallic hydrogen chemically. His reasoning was simple: For a long time it was believed that the diamond could only be obtained from the gigantic pressures found inside the planets, but today there are already technologies to cultivate diamond crystals, making them grow layer by layer using a known technique as chemical vapor deposition.
The team then found that the addition of carbon and sulfur “cheats” hydrogen, making it behave as if the pressure were much higher than it actually is – obtaining metallic hydrogen in the laboratory is still a major target issue. controversies among scientists.
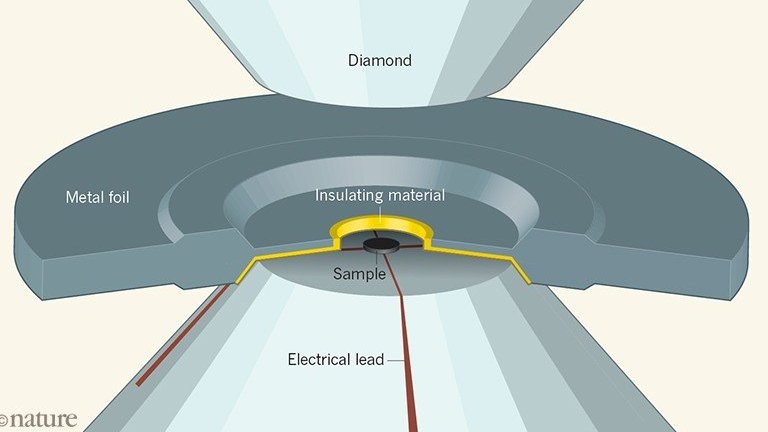
“To have a high temperature superconductor, you want stronger bonds and lighter elements. Those are the two basic criteria. Hydrogen is the lightest material and the hydrogen bond is one of the strongest,” explained Professor Ranga Dias, team coordinator.
Superconductivity at room temperature
It worked when they found the right combination of elements, with their hydrogen, carbon and sulfur compound showing superconductivity at 15 ° C, which can be well accepted as “room temperature” – at the beginning of last year, another North American team American had reached superconductivity at -13 ° C .
The big drawback to our long-awaited energy revolution is that superconductivity only worked inside a diamond anvil, an apparatus designed to achieve very high pressures.
In this experiment, the compound became superconducting at about 267 gigapascals, or 39 million PSI, or 2.6 million atmospheres, which is about 70% of the pressure found in the Earth’s core.
The team will now dedicate itself – together with the entire world scientific community – to trying to understand the chemical structure of its compound, a carbonaceous sulfur hydride. In fact, they do not know how hydrogen, carbon and sulfur are structured under this pressure, and understanding this is essential so that they can gradually remove the pressure without losing superconductivity.
“We live in a semiconductor society and, with this type of technology, you can take the society to a superconducting society, where you will never need things like batteries again,” predicts Professor Ashkan Salamat, a member of the team.
Article: Room-temperature superconductivity in a carbonaceous sulfur hydride
Authors: Elliot Snider, Nathan Dasenbrock-Gammon, Raymond McBride, Mathew Debessai, Hiranya Vindana, Kevin Vencatasamy, Keith V. Lawler, Ashkan Salamat, Ranga P. Dias
Magazine: Nature Physics
Vol .: 586, pages 373-377
DOI: 10.1038 / s41586-020-2801-z































Discussion about this post