Helium inaugurates the group of rare gases in the periodic table . Almost inert, it is the second most abundant element in the universe after hydrogen.
It is the second lightest element. The world’s primary source of helium is a group of natural gas fields in the United States.
History of Helium
The first known manifestation of helium was evidenced by a line at 547.49 nanometers in the spectrum of the solar chromosphere in 1869 by Jules Janssen, wrongly attributed to sodium . The same year, Norman Lockyer in turn observed a line in the solar spectrum, which he attributed to an element unknown on Earth . Edward Frankland and the Norman Lockyer named after Helios , the Greek word for sun . It was in 1895 that Norman Ramsay isolated helium on Earth.
Properties of Helium
Helium is transparent , odorless and tasteless or poisonous gas. It is less soluble in water than any other gas. It is also the least reactive element and essentially does not form chemical compounds.
The density and viscosity of helium vapors are very low while the thermal conductivity and specific heat are very high.
It is possible to liquefy helium, but its condensation temperature is the lowest among all known substances.
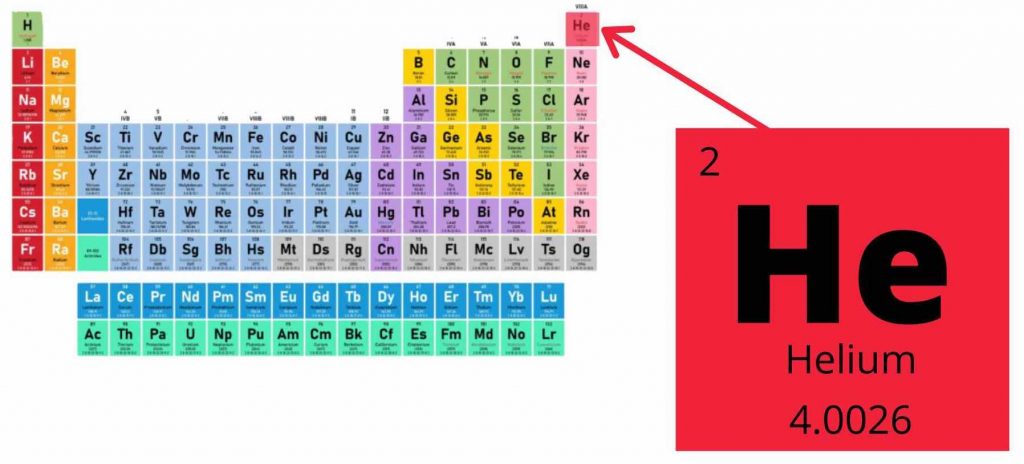
What is Helium used for?
Helium was the first gas used to fill balloons and airships, and it is still used for weather balloons in particular.
The main use of helium is the inert shielding gas in autogenous welding. Its greatest potential is found in applications at very low temperatures. Helium is the only refrigerant capable of reaching temperatures below 14 K (-434ºF). It remains in the gaseous or liquid state.
It is used as pressurizing gas for rocket fuels, in diving cylinders mixed with oxygen , in the cooling of certain nuclear reactors or in chromatography.
On Earth, helium is formed by radioactivity from heavier elements. Once on the surface, helium migrates through the atmosphere before escaping at about the same rate as it appears on the surface.
Effects of Helium on health
- On exposure: The substance can be absorbed into the body by inhalation.
- Inhalation: Elevated voice. Dizziness Heaviness. Headache.
- Suffocation. Skin Freezing in contact with liquid.
- Inhalation risk: If there are leaks in the container, this gas can cause suffocation, since it reduces the oxygen content in the air in closed places.





























Discussion about this post