What is Platinum?
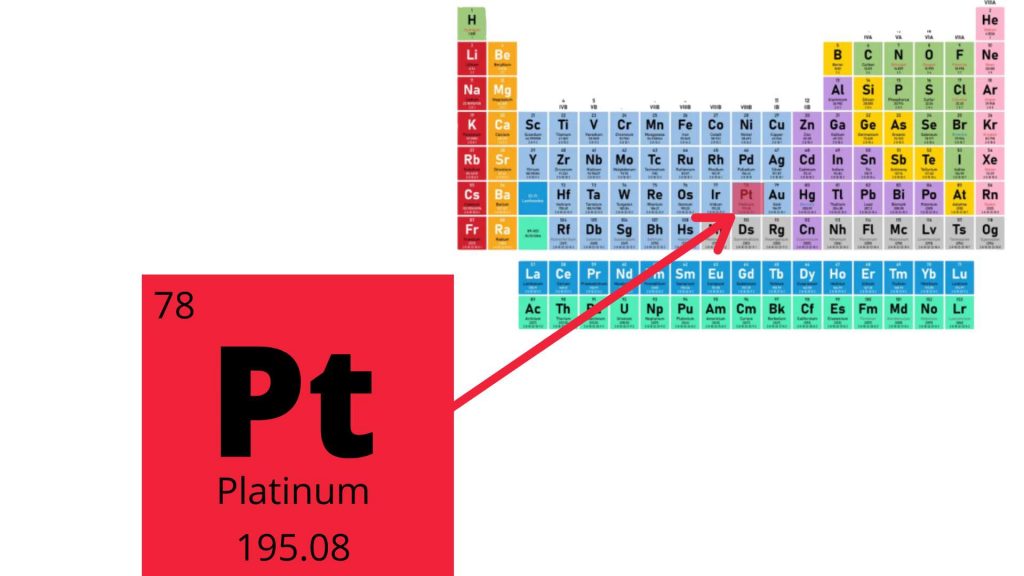
Platinum is a chemical element denoted as Pt and atomic number 78. It is a precious metal characteristic with high density, ductile, gray-white transition metal . It has remarkable corrosion resistance.
Platinum is commonly used to make jewelry , automotive catalysts, laboratory equipment , dental implants and contact materials. Because of its rarity, only small amounts are extracted globally. Some platinum-containing complexes such as cisplatin are used in chemotherapy for certain types of cancer. It is a little reactive metal and is often found in its metallic form.
Properties of Platinum
| Element Category | Transition metal | Density | 21.45 g/cm3 |
| Physical Form (at 20°C) | Solid | Boiling Point | 4098 K (3825 °C, 6917 °F) |
| Atomic Number | 78 | Melting Point | 2041.4 K (1768.3 °C, 3214.9 °F) |
| Group | Group 10 | Vaporization Heat | 510 kJ/mol |
| Period | Period 6 | Fusion Heat | 22.17 kJ/mol |
| Electron Configuration | [Xe] 4f145d96s1 | Molar Heat Capacity | 25.86 J/(mol·K) |
| Thermal Expansion | 8.8 µm/(m·K) (at 25 °C) | Thermal Conductivity | 71.6 W/(m·K) |
| Electrical Resistance | 105 nΩ·m (at 20 °C) | Crystal Structure | FCC |
| Poisson ratio | 0.38 | CAS Number | 7440-06-4 |
| Vickers hardness | 400–550 MPa | Tensile strength | 125–240 MPa |
| Brinell hardness | 300–500 MPa | Young’s modulus | 168 GPa |
| Mohs hardness | 3.5 | Shear modulus | 61 GPa |
| Bulk modulus | 230 GPa | Atomic Weight | 195.084 |
History – Bad Childhood of Platinum
The name is derived from the Spanish word platina. The first mention comes from the Italian humanist Julius Caesar Scaliger . He describes platinum as a mysterious white metal that eluded all attempts at melting. A more detailed description of the properties can be found in a report by Antonio de Ulloa published in 1748.
In the 17th century, platinum became a major problem in the Spanish colonies as an annoying material to search for gold. It was thought to be “immature” gold and was thrown back into the rivers of Ecuador . Since it has a similar specific weight to gold and did not tarnish even in the fire, it was used to adulterate gold.

The Spanish government then issued an export ban. They even considered sinking all of the platinum that had been preserved so far to prevent and deter platinum smuggling and counterfeiting.
Louis Bernard Guyton de Morveau found a simple process in 1783 for the industrial production of platinum.
Is Platinum better than gold?
Do you know only 160 tons of platinum are mined annually compares 1500 tons of gold. As it is rarer and mined much less than gold, Platinum held as expensive metal. Also, platinum is denser than gold, so the ring will weigh significantly more in platinum made than in gold.
Of all this, the honest answer is one is better than other depends on application. Platinum is far more valuable if you are a manufacturer of catalytic converters for cars.

What is the use of platinum?
Platinum is also used in an many number of areas like below,
- Platinum is a favorite material for the production of laboratory equipment because it does not produce flame coloring . There are e.g. B. thin platinum wires are used to hold samples in the flame of a Bunsen burner.
- Platinum is a noble and valuable metal – It is almost sixty times more expensive than silver. It has been used as xpensive jewelry and Premium pens, as well as cash or investment use.
- Medical implants, alloy additive in dental materials.
- Thermocouples
- Magnetic materials
- Coatings of turbine guide vanes in aircraft engines
- Resistance thermometer
- Heating resistors
- Contact materials and electrodes, e.g. B. in spark plugs
- Catalysts
- Chemical apparatus construction, laboratory and analysis devices
- Melting pot for glass production
- Pacemaker
- Thrusters, rocket linings
- Spinnerets
- Platinum mirrors (mirrors and semitransparent mirrors which, unlike silver mirrors, cannot tarnish)
- Laser printer (charging corona)






























Discussion about this post